Silane Coupling Agents in Dentistry: Chemistry, Activation, and Clinical Protocols
1. What Is Silane?
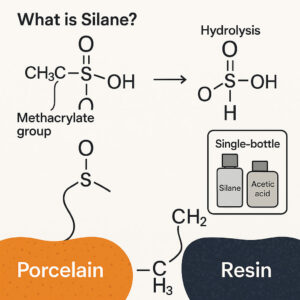
Silane is an organosilicon compound containing one or more silicon atoms bonded to organic functional groups.
The type of silane used in dentistry is bifunctional. One end of the molecule contains a methacrylate group, which can bond to methacrylate-based adhesives and resin cements, while the other end, after hydrolysis, reacts with the hydroxyl groups on the porcelain surface to form strong covalent bonds.
Silane is a molecular bridge between inorganic substrates (ceramics) and organic resin matrices, enabling durable adhesion between two otherwise incompatible materials. (Magne & Belser, Bonded Porcelain Restorations in the Anterior Dentition, 2002)
2. Activation of Silane (Hydrolysis)
For silane to effectively bond, it must undergo hydrolysis, converting alkoxy groups (–OR) into silanol groups (–Si–OH), which can then react with the silica phase of ceramics.
This activation is typically achieved using acetic acid as a mild catalyst, lowering the pH to around 4–5 and promoting controlled hydrolysis.
3. Types of Silane Systems in Dentistry
A. Single-Bottle (Prehydrolyzed) Silanes
-
Contain 1–5% pre-hydrolyzed silane in an ethanol or water solution with acetic acid.
-
pH ranges between 4 and 5.
-
Advantages: Ready-to-use, convenient, no mixing required.
-
Drawbacks: Limited shelf life—once hydrolyzed, silane molecules tend to self-condense, forming oligomers with reduced reactivity and bond strength.
-
Must be refrigerated and brought to room temperature before use.
-
Discard if the solution becomes cloudy, milky, or shows sediment, indicating polymerization and loss of activity.
-
Shelf life: ~12 months (per Rosenstiel et al., Contemporary Fixed Prosthodontics).
B. Two-Bottle Silane Systems
-
One bottle contains unhydrolyzed silane, and the other contains acetic acid for activation.
-
These are mixed immediately before being used to produce fresh, active silane.
-
Depending on the manufacturer and study, recommended waiting periods after mixing vary from 10 to 60 minutes.
Some research suggests the reaction continues on the porcelain surface, allowing immediate use. -
Advantages: Long shelf life, consistent activity.
-
Critical: Always follow the manufacturer’s activation and mixing instructions precisely.
4. Clinical Application Guidelines
a. Amount and Layer Thickness
Apply only a thin, uniform layer—usually one coat is sufficient.
Excess silane forms a thick polymerized layer that behaves as if it were mechanically weak and may compromise bond durability.
If the surface appears glossy after drying, this indicates overapplication; the surface should be re-sandblasted and re-etched.
b. Heat Application (Hot Air Drying)
Research (Blatz et al., J Adhes Dent, 2003) shows that heating silanized surfaces significantly enhances bond strength.
After applying silane, blow warm air (≈38–50°C) for 60 seconds to:
-
Evaporate solvents (water, ethanol).
-
Promote condensation reactions between silane and ceramic.
-
Improve interfacial siloxane bonding and long-term durability.
c. Recommended Protocol
-
Etch porcelain with HF acid (per ceramic-specific time).
-
Clean and dry thoroughly.
-
Apply one coat of silane, air dry for 60 seconds with warm air.
-
(Optional) Apply a second coat and repeat the warm air step.
-
Proceed immediately with resin adhesive application.
5. Mechanism of Bonding
Silane forms Si–O–Si covalent bridges between the silica in ceramics and the methacrylate groups in resin cements.
This dual reactivity creates chemical coupling and improved wettability, essential for high bond strength and hydrolytic stability.
6. Storage and Handling Recommendations
-
Refrigerate single-bottle silanes; discard after 12 months.
-
Mix two-bottle systems immediately before use.
-
Avoid contamination by water or light exposure.
-
The material should be replaced if the appearance changes (cloudiness, separation).
Conclusion
Silane coupling agents remain indispensable in adhesive ceramic dentistry, bridging the gap between inorganic and organic phases.
While prehydrolyzed (single-bottle) silanes offer convenience, freshly activated two-bottle systems provide superior chemical stability and performance.
When combined with proper HF etching and thermal activation, silane application results in durable, high-strength bonds crucial for the longevity of indirect ceramic restorations.